COVID-19 Vaccine Questions and Answers for Healthcare Professionals
This Q&A aims to provide clear and comprehensive answers to common COVID-19 vaccine questions. This page will benefit healthcare professionals who are looking for tools to explain technical information about vaccination to their patients more effectively. Answers are presented in two parts: key messages expressed in plain language that address the question at hand, and a more technical-oriented summary that dives deeper into the supporting evidence.
If you have a question, please contact us and we will review them for inclusion to the question list.
Multiple Languages: This page is also available in French, Chinese, Arabic, and Spanish (last updated: December 16, 2021)
Disclaimer: As the science and public health guidance around COVID-19 vaccines are rapidly evolving, there is no guarantee that the information provided in this page is complete, and up-to-date. However, as more questions come to our attention or more information becomes available, the answers will be updated and new answers will be posted. The questions and answers are hosted on this page and are cross-referenced on other sites.
COVID-19 Vaccine Safety Q & A
General
Is a COVID-19 vaccine needed after a COVID-19 infection?
COVID-19 vaccination is highly recommended post-infection.
What You Need to Know
- People who have had a COVID-19 infection are strongly advised to receive a COVID-19 vaccine dose.
- Protective immunity gained from a COVID-19 infection is not enough to protect against reinfection with COVID-19 nor against serious disease from it.
- There are conflicting recommendations on how long to wait before receiving a COVID-19 vaccine dose following a COVID-19 infection.
- COVID-19 “hybrid immunity” (immunity gained from infection plus vaccination) offers better protection than infection alone.
COVID-19 Vaccine Recommendations After Infection
As more and more people have contracted COVID-19, both in Canada and worldwide, people are wondering if and when COVID-19 vaccines should be taken following an infection with COVID.
Currently, major health authorities around the world all agree that people who have had a COVID-19 infection are strongly advised to receive a COVID-19 vaccine dose (initial doses or booster dose). There are differing recommendations for how long one should wait after a COVID-19 infection:
- The National Advisory Committee on Immunization (NACI) in Canada recommends waiting 6 months before getting a COVID vaccine following a COVID-19 infection.
- The World Health Organization (WHO) also recommends delaying vaccination for 6 months following a COVID-19 infection.
- The United States Centers for Disease Control and Prevention (CDC) recommends only a 3-month wait.
These differences may have to do with the way we prioritize how quickly we want to be protected versus how long the protection will last. Receiving a COVID-19 vaccine only 3 months after a COVID-19 infection will indeed protect an individual much sooner. Then again, being vaccinated after a 6-month wait can result in more long-term protective immunity against the virus. Unfortunately, we are currently lacking scientific studies to confirm the best timing for COVID-19 vaccination after a confirmed COVID-19 infection. Nonetheless, those at higher risk of serious COVID-19 disease should receive a COVID-19 vaccine dose as rapidly as possible, based on their specific risk and context. This means that for some, the 3-month wait may be the best option, while for others the 6-month delay is best.
Protective Immunity After a COVID-19 Infection
Natural infection is not a perfect way to gain lasting protection against COVID-19 disease. Although being infected with COVID-19 does provide some protective immunity, it does not protect against all existing variants of the virus. Infection with COVID-19 a second (and even a third) time has occurred. The amount of protective immunity gained following a COVID-19 infection depends on the COVID-19 variant that caused the infection, the degree of infection, and whether the person had received a COVID-19 vaccine dose prior to infection (including the type of vaccine and when it was received).
The protective immunity developed from a COVID-19 infection does not necessarily protect against a second infection, nor does it protect you from infection even when you’re vaccinated (medically known as a “breakthrough infection”). In fact, the protective immunity gained from an infection in combination with vaccination (called “hybrid immunity”) offers better protection than infection alone.
More Information for Healthcare Providers
Here are additional considerations for when to offer a COVID-19 vaccine after a documented COVID-19 infection. Each patient must be assessed based on their individual risk. Several factors need to be considered in deciding when a COVID-19 vaccine dose should be given following a confirmed COVID-19 infection, such as:
- The person’s age
- Whether the person is immunocompromised (due to health conditions or medications)
- The date of their last COVID-19 vaccine dose
- Their risk of exposure, for example, whether the COVID infection rate is currently high in their community
- Whether they are in contact with someone or people at higher risk for serious COVID-19 infection
- How easily the individual can have access to the COVID vaccines
References
Amirthalingam, G., Bernal, J.L., Andrews, N.J., Whitaker, H., Gower, C., Stowe, J. et al. 2021. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nature Communications, 12(1):7217. 10 December 2021. https://doi.org/10.1038/s41467-021-27410-5
Hulme, W.J., Williamson, E.J., Horne, E., Green, A., Nab, L., Keogh, R., et al. 2022. Effectiveness of BNT162b2 booster doses in England: an observational study in OpenSAFELY -TPP. medRxiv. 6 June 2022. https://doi.org/10.1101/2022.06.06.22276026
Ireland, G., Whitaker, H., Ladhani, S.N., Baawuah, F., Subbarao, V., Linley, E. et al. 2021. Serological responses to COVID-19 booster vaccine in England. medRxiv. 24 November 2021. https://doi.org/10.1101/2021.11.22.21266692
National Advisory Committee on Immunization (NACI). 2022. Recommendations on the use of bivalent Omicron-containing mRNA COVID-19 vaccines. An Advisory Committee Statement (ACS). 1 September 2022. https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-bivalent-Omicron-containing-mrna-covid-19-vaccines.pdf
Shaheen, N.A., Sambas, R., Alenezi, M., Alharbi, N. K., et al. 2022. COVID-19 reinfection: A multicenter retrospective study in Saudi Arabia. Ann Thorac Med, 2022 Apr-Jun; 17(2): 81-86. doi: 10.4103/atm.atm_74_22.
Skowronski, D.M., Setayeshgar, S., Febriani, Y., Ouakki, M. et al. 2021. Two-dose SARS-CoV-2 vaccine effectiveness with mixed schedules and extended dosing intervals: test-negative design studies from British Columbia and Quebec, Canada. medRxiv. 26 October 2021. doi: 10.1101/2021.10.26.21265397.
World Health Organization (WHO). 2022. Interim statement on hybrid immunity and increasing population seroprevalence rates. 1 June 2022. https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates
Can mRNA vaccines “genetically modify” humans?
mRNA vaccines cannot make changes to human DNA.
What You Need to Know
- The COVID-19 mRNA vaccine cannot change human DNA
- The COVID-19 mRNA vaccine is not a live-virus vaccine
- The COVID-19 mRNA vaccine teaches your immune system how to identify the virus in order to fight it
- The COVID-19 mRNA vaccine does not create anything that is harmful to you
- The mRNA and spike protein are naturally destroyed after completing their job
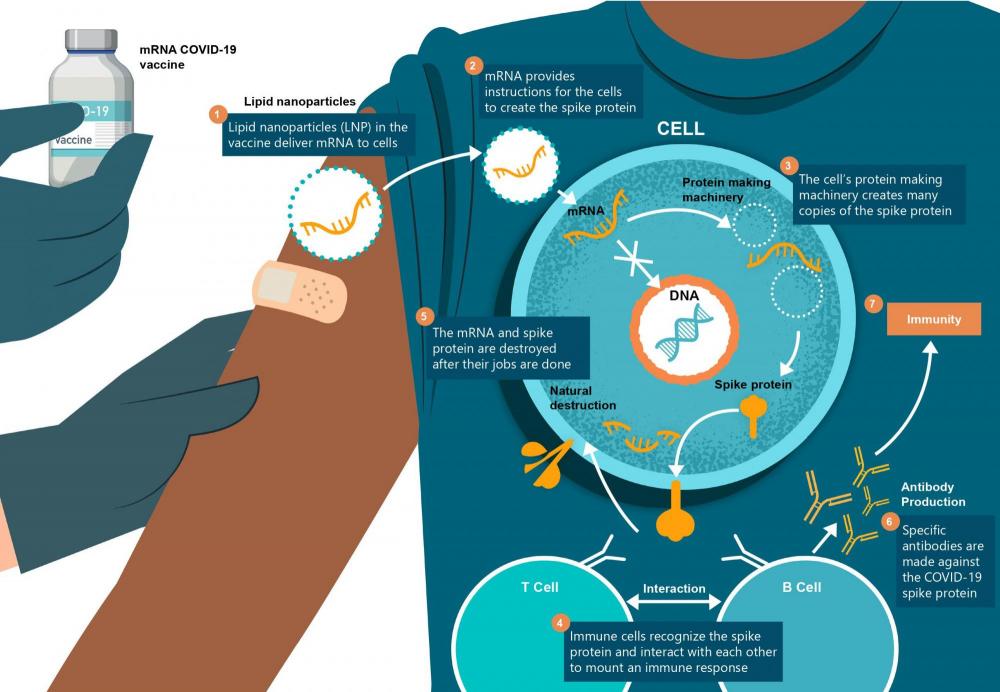
How mRNA COVID-19 vaccines work
Messenger RNA—also known as mRNA—provides the instructions your cells need to fight the virus that causes COVID-19. These instructions tell your cells to produce a harmless piece of the virus, in this case, what’s known as the “spike protein.” This same protein is naturally found on the outside of COVID-19 viruses. The mRNA vaccine does not provide instructions for producing the whole virus.
By instructing your cells to produce the spike protein, the mRNA vaccine teaches your immune cells to recognize it as foreign to your body. This triggers immune cells to become active. They will interact with other immune cells to mount a response to fight off what appears to the immune system as an infection. Your immune system will remember how to recognize the virus to better protect your body against any future COVID-19 infection.
The benefits of COVID-19 vaccination
COVID-19 vaccines get your body ready to fight a COVID infection if you are exposed to the real virus in the future. The benefit of vaccination is that you gain protection against the virus without risking the potential danger of getting sick from COVID-19.
More information for healthcare providers
COVID-19 mRNA vaccines are not live-virus vaccines. Instead, they contain synthetic pieces of messenger RNA that are encapsulated by an engineered Lipid Nanoparticle (LNP). The LNP allows the mRNA to be delivered directly into the host cell and provides a stabilization and adjuvant effect. As a result, these vaccines do not require additional adjuvant to enhance their efficacy.
Once inside the host cell of the human recipient, the mRNA does not enter the nucleus and does not integrate itself into human DNA or cause any alterations to human DNA.
The COVID-19 mRNA vaccine primes the immune system to better prepare the recipient against a future COVID-19 infection. It provides instructions that allow human cells to make the spike protein that is found on the surface of the COVID-19 virus. Once the spike protein is made, it ends up on the surface of the cell that made it. The immune system then recognizes that the protein does not belong to the cell and activates immune cells to produce specific antibodies. Both B cells and T cells are involved in this response. This is the same response that would occur during a natural COVID-19 infection. As a result, the recipient’s immune system is trained to respond to a future COVID-19 infection.
After the spike protein is made and presented on the surface of cells, both the mRNA and spike proteins are naturally degraded within a few days of injection. The mRNA does not replicate, and eventually disappears.
References
Understanding mRNA COVID-19 vaccines. Centres for Disease Control and Prevention. Nov 23, 2020. Accessed on 14 Dec 2020 at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html.
Advances in mRNA vaccines for infectious diseases. Zhang C, Maruggi G, Shan H, Li J. Front Immunol. 2019; 10: 594. doi: 10.3389/fimmu.2019.00594.
Developing mRNA-vaccine technologies. Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. RNA Biol. 2012; 9(11): 1319-1330. doi:10.4161/rna.22269
mRNA vaccines – A new era in vaccinology. Pardi N, Hogan MJ, Porter FW, Weissman D. Nat Rev Drug Discov. 2018; 17(4):261-279. doi:10.1038/nrd.2017.243.
Are there safety concerns with mRNA vaccines?
mRNA vaccines are safe.
What You Need to Know
- mRNA vaccines are not new
- mRNA vaccines have been well studied and are safe
- mRNA vaccines do not affect your DNA
- mRNA vaccines can be produced rapidly and safely
The COVID-19 mRNA Vaccine Is Not the First of Its Kind
mRNA vaccines aren’t new! In fact, mRNA vaccines have been used and studied for Zika virus, rabies, influenza, and cytomegalovirus, as well as for cancer treatments. They are currently a hot topic because they represent a huge advancement in the field of vaccines. Unlike other vaccines, mRNA vaccines do not require adjuvants—chemicals that make vaccines more effective—to do their job. This means that mRNA vaccines do not pose a concern for unwanted reactions after being injected. They have also gained a lot of attention because of the benefits they have provided during the current COVID-19 pandemic.
mRNA Vaccines are Safe and Effective
Using mRNA in vaccines is a safe and effective way to train the immune cells in your body to fight viruses. They teach your immune system to specifically recognize and fight what does not belong in your body.
mRNA vaccines can also be rapidly produced. In comparison, other types of vaccines usually take months to produce. This has been a great advantage during the COVID-19 pandemic.
mRNA tends to be delicate and must be stored and handled with great care. This means that mRNA vaccines must be kept frozen and used shortly after thawing.
More Information for Healthcare Providers
mRNA vaccines have been previously studied for Zika, rabies, influenza, and cytomegalovirus (CMV) vaccination. They have also been studied in cancer research as a way to trigger the immune system to target specific types of cancer cells. mRNA is a highly attractive solution to diseases since it allows for any protein to be expressed by the human body from engineered, synthetic mRNA. This allows for a more targeted approach than that of some adjuvant-dependent vaccines that mount a more general immune response. There are other advantages to using mRNA vaccines, yet we still need to address some basic limitations.
Advantages
- mRNA is a safe vector for a vaccine as it is transient (i.e., it decays metabolically within a few days) and a carrier of information (i.e., protein-making instructions) that does not interact with the vaccine recipient’s DNA. Moreover, mRNA is non-replicative, that is, it cannot make copies of itself.
- Soon, mRNA technology could potentially allow for one vaccine to provide protection against multiple diseases. This would decrease the number of injections required for protection and possibly increase overall vaccination rates for greater herd immunity.
- mRNA manufacturing and Lipid Nanoparticle (LNP) formulation processes are fast and highly scalable, which enables a high volume of vaccine doses to be produced rapidly and safely. Rapid production allows us to be more responsive to viral outbreaks. As a result, mRNA vaccines are highly suitable for rapid vaccine development in a pandemic context.
Limitations
- Due to the fragile composition of mRNA, there is a need for very careful storage and handling of mRNA vaccines to maintain their stability.
Here are the storage and handling requirements for approved COVID-19 vaccines in Canada:
- Pfizer mRNA COVID-19 vaccine (BNT162b2): stored between -80°C and -60°C (112°F to 76°F). After thawing and dilution prior to administration, it must be stored between 2°C and 25°C (35°F to 77°F) and used within 6 hours from the time of dilution.
- Moderna mRNA COVID-19 vaccine (mRNA-1273): stored at -20°C (-4°F) for up to 6 months. After thawing, at 2° to 8°C (36° to 46°F) for up to 30 days within the 6-month shelf life.
References
Understanding mRNA COVID-19 vaccines. Centres for Disease Control and Prevention. Nov 23, 2020. Accessed on 14 Dec 2020 at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html.
Advances in mRNA vaccines for infectious diseases. Zhang C, Maruggi G, Shan H, Li J. Front Immunol. 2019; 10: 594. doi: 10.3389/fimmu.2019.00594.
Developing mRNA-vaccine technologies. Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. RNA Biol. 2012; 9(11): 1319-1330. doi:10.4161/rna.22269.
mRNA vaccines – A new era in vaccinology. Pardi N, Hogan MJ, Porter FW, Weissman D. Nat Rev Drug Discov. 2018; 17(4): 261-279. doi:10.1038/nrd.2017.243.
Clinical Trials
How is vaccine safety determined?
Vaccines undergo rigorous studies to test their safety and efficacy.
What You Need to Know
- Vaccines require a higher proof of safety than most drugs
- Vaccine safety is thoroughly tested in pre-clinical studies and clinical trials
- The COVID-19 vaccines are no exception and have followed the same processes to test safety and efficacy
- Vaccines are evaluated by unbiased government regulators and non-government experts for final approval
All Vaccines Need Proof of Safety and Effectiveness
Vaccines are given to healthy people to prevent disease. That is why vaccines require a higher proof of safety than most therapeutic drugs. They must follow very stringent evaluations using a group of people who have volunteered as test subjects before they are used in the wider population. Vaccines are ultimately approved by government regulators and non-government experts as safe and effective based on the results of these safety and effectiveness tests. The people who approve vaccines do not work for the companies that make them. If the vaccines do not meet safety and effectiveness standards during these tests, they will not be approved.
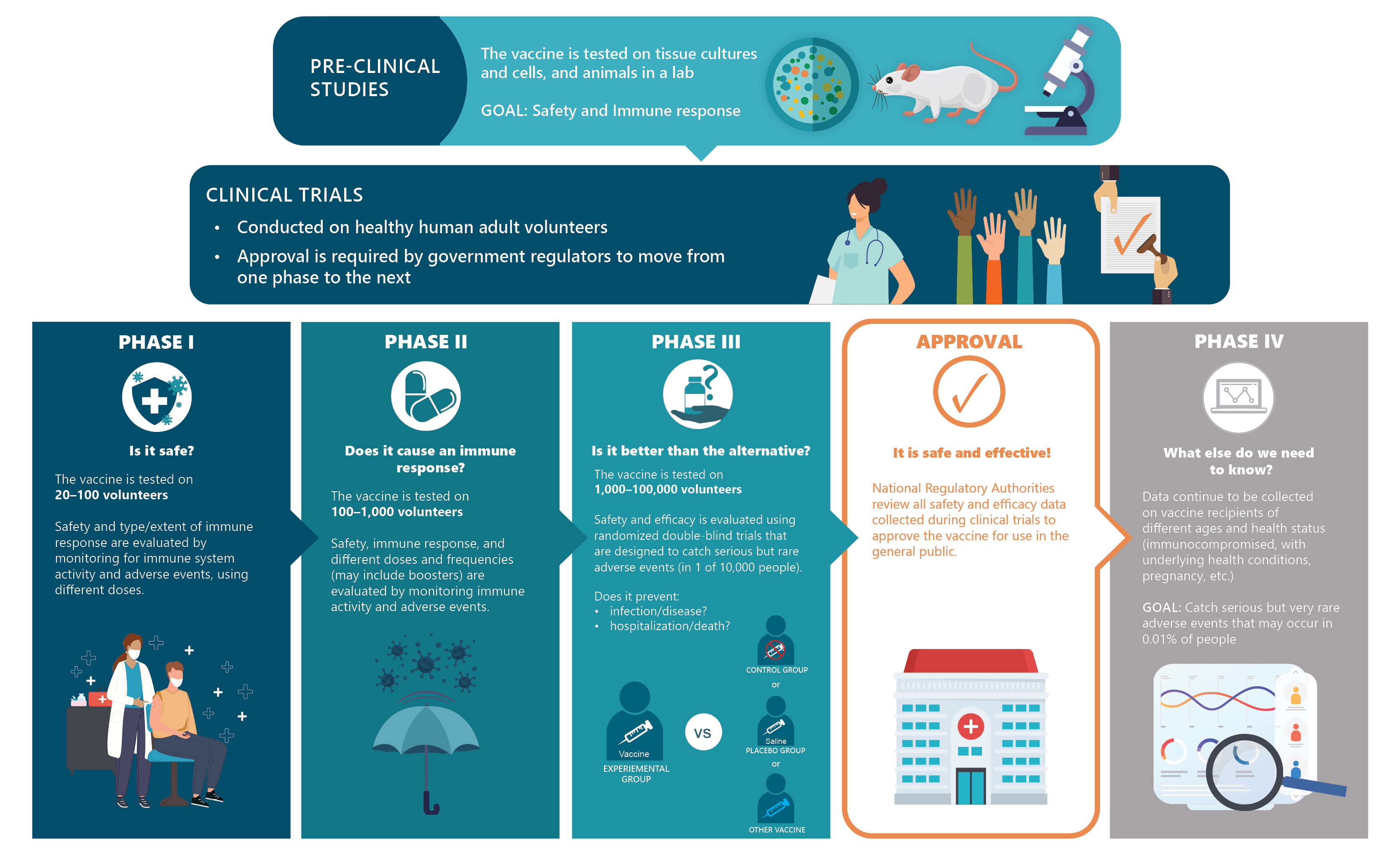
Vaccines Must Pass Pre-Clinical Studies and Clinical Trials
During testing, vaccines must show two things—that they are safe (they cause no harm to people) and effective (they protect against a virus). Vaccines are evaluated in 4 steps. A vaccine cannot move onto the next step unless it is safe.
The first step, known as a pre-clinical study, tests the vaccine in the laboratory without using human volunteers. The next three steps are known as clinical trials, since they involve human volunteers.
- Pre-clinical studies: this first step studies the vaccine using tissues, cells, or animals (like mice) in the laboratory.
- Phase I clinical trials: if the vaccine passes the first step, it is then tested on a small group of adults (about 20 to 100 volunteers).
- Phase II clinical trials: if the vaccine passes Phase I, it is further tested on a larger group of adults (hundreds of individuals).
- Phase III clinical trials: if the vaccine passes Phase II, it is then tested on a much larger group of adults (thousands to tens of thousands of individuals).
Information from these studies and trials is then further evaluated by government regulators and non-government experts for approval. Only when a vaccine has passed through all these steps can it be approved for use in Canada.
COVID-19 Vaccines Are No Exception
A common misconception is that COVID-19 vaccines were produced too quickly to have passed the required safety and efficacy steps, and that not enough data was collected to deem them safe. This is not correct. All approved COVID-19 vaccines have gone through all of the standard safety and efficacy procedures and processes before being given to Canadians. In addition, millions of people around the world have now received doses of COVID-19 vaccines that are approved for use in Canada. This means that we have a large amount of data—much larger than compared to other vaccines—to support that mRNA COVID-19 vaccines are safe.
More Information for Healthcare Providers
Vaccine development undergoes a rigorous set of carefully implemented scientific and ethical processes and procedures to ensure the safety and efficacy of vaccines before they are introduced into populations. At each stage, the data from the studies and trials are assessed by government regulators to give permission to move to the next stage. The series of studies and trials goes as follows:
Pre-clinical: Studies are completely in a laboratory setting to evaluate both the vaccine’s safety and immunogenicity (i.e., its ability to produce an immune response) by using tissue-culture, cell-culture, and/or animal models (e.g., mice, ferrets, and/or monkeys).
Clinical Trials: Clinical trials are conducted on healthy human adult volunteers in three distinct phases. Approval is given by government regulators at each phase before moving onto the next.
Phase I: These clinical trials aim to assess the safety of the vaccine and the type and extent of immune response in a small group of test subjects.
Phase II: These clinical trials assess the vaccine’s safety, immunogenicity, dosing, and schedule using a larger group of volunteers.
Phase III: These clinical trials further assess safety and efficacy through randomized and double-blind trials where the vaccine is tested against a control group (i.e., where no injection is given) or placebo group (i.e., where an inert substance, such as a saline injection, is given). Phase III clinical trials allow for the detection of rare side effects that are too infrequent to be observed in smaller groups (e.g., in fewer than 1 to 10/10,000 individuals).
Based on these studies and trials, the vaccine’s safety and efficacy are then evaluated by government regulators (independent from the researchers who conducted the studies and the vaccine’s manufacturer) and by non-government experts before being approved for use in Canada. The COVID19 vaccines are no exception and have been put under the same rigorous set of evaluations, even though they were produced rapidly through mRNA technology.
References
Canada’s eight-component vaccine safety system: A primer for health care workers. MacDonald NE, Law BJ. Paediatrics & Child Health, 2017, e13-16. Accessed on 28 Sep 2020 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5825834/pdf/pxx073.pdf.
Vaccine Testing and Approval Process. Centers for Disease Control and Prevention. Accessed on 25 Sep 2020 at https://www.cdc.gov/vaccines/basics/test-approve.html.
Accelerating a safe and effective COVID-19 vaccine. World Health Organization. Accessed on 25 Sep 2020 at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/accelerating-a-safe-and-effective-covid-19-vaccine.
What measures are used to ensure vaccine safety?
Laws, regulations, and guidelines are used to ensure that vaccines are safe and effective.
What You Need to Know
- Vaccines must pass national and international standards for approval
- In Canada, vaccines are approved by National Regulatory Authorities like Health Canada
- Despite the urgency, Canadian National Regulatory Authorities did not take shortcuts with COVID-19 vaccines
- Clinical trials are overseen by Research Ethics Boards to ensure that research is done ethically
- International bodies oversee regulatory obligations and research in humans for safety, effectiveness, and quality
- Vaccine safety surveillance continues after clinical trials, and is ongoing once vaccines are in regular use
Who Does What?
There are laws, regulations, and guidelines at the national and international levels that ensure that the vaccine is safe and works properly. Three different types of authorities ensure that processes to ensure the safety and effectiveness of vaccines are followed.
1. Institutional Research Ethics Boards. These organizations, known as IRBs or REBs, decide if vaccine research protocols are ethical. In Canada all clinical trials must be registered with the IRB known as the Tri-Council Panel on Research Ethics.
Clinical trials must meet the following ethical standards:
- Informed consent: ensures the freedom of individuals to make choices about their medical care.
- Decision-making capacity: ensures that research participants can make meaningful decisions when participating in a study.
- Trial conduct: ensures that the vaccines are being safely tested in human volunteers and that participants’ information are handled properly.
- Fairness and equity: ensure that research participants do not bear an unfair share of the direct burdens by participating in research, while not being unfairly excluded from the potential benefits.
- Privacy and confidentiality: ensure that clinical trial participants’ medical information and data is collected, stored, used, and disclosed in a manner that protects their privacy.
- Conflict of interest: ensures that there are no financial or other personal considerations that may compromise, or have the appearance of compromising, a researcher’s professional judgment when conducting and reporting research.
- Safety monitoring and reporting: ensure that reports and collected information on actual and potential safety issues are properly filed.
2. National Regulatory Authorities. These organizations are responsible for approving clinical trials from Phase I to II, and then to Phase III. They are also responsible for thoroughly evaluating all of the evidence from these trials before approving a vaccine in their respective countries. Each clinical trial must have a Data Safety Monitoring Board. This group of experts examines safety-related data while clinical trials are taking place. Their job is to pause trials when an adverse event occurs. They also have the power to completely stop a clinical trial if they deem it unsafe.
3. International bodies oversee research in humans and ensure that regulations for safety, effectiveness, and quality are being followed. The World Health Organization (WHO) also coordinates expert advisory panels that deliberate on matters of global health, provide ethical guidance, and work with National Regulatory Authorities to license safe vaccines in low-income countries.
Transparency is Key!
Transparency—making it easy for others to see what actions are being taken and why—throughout the entire vaccine research and approval process is critical for safety and properly informing the public. This includes full disclosure of trial protocols, data and technical documents, clinical study reports, stopping rules, and decision-making.
The COVID-19 pandemic created an urgent need for vaccines. Such public safety emergencies can sometimes be used to speed up the vaccine regulatory process and to authorize emergency use of a vaccine. Faster approvals require the utmost vigilance to ensure that safety is never compromised. In the case of COVID-19, National Regulatory Authorities did not take any shortcuts in approving vaccines.
More Information for Healthcare Providers
COVID-19 vaccine safety is ensured through regulatory and legal standards that limit conflicts of interest, enforce manufacturing quality standards, and make it mandatory to report serious Adverse Effects Following Immunization (AEFIs). This includes:
- Clear disclosure guidelines that exclude those with possible personal and/or professional conflict of interest from being involved in decision-making.
- Transparency and open access to all trial protocols, data (including vaccine components, such as adjuvants), amendments and adaptations, and decision-making processes and rationales.
- Modifications, like those that either compress testing phases or add new clinical testing steps due to new findings, being made to clinical trials if there is no increased risk to participants. Any modifications must be made immediately known with rationale and data/evidence to support those decisions.
- All clinical trial evidence and decision-making being independently appraised in an unbiased manner. This includes advisory committee recommendations and vaccine promotion to address perceived conflicts of interest.
- Clinical trials must have sufficient statistical power to ensure safety as well as efficacy. For instance, Phase III clinical trials can miss rare but serious adverse events if the number of participants is too small (e.g., an occurrence rate of ≥ 0.01% and < 0.1%). Large trial populations are needed to identify such rare adverse events that may result in morbidity (e.g., intussusception with Rotashield).
- Post-approval medical monitoring and active medical surveillance must be carried out to detect very rare but serious adverse events following vaccination, including detection of vaccine failures.
- Actual disease prevention—lowering the rate of actual COVID-19 infections—rather than relying on surrogate endpoints that are indirect evidence of an infection. For example, the visible agglutination of red blood cells is often used to indicate the binding of antibodies to antigens on the red blood cell surface. This is known as the serological method which shows the presence or absence of antibodies that are specific to a pathogen but does not prove whether there is an active infection.
- Phase III trials being conducted in locations where the disease is prevalent to accurately represent disease exposure and to properly determine disease prevention.
- Human challenge trials—where subjects are intentionally exposed to a pathogen—being unethical since there is currently no available cure for COVID-19.
- Under-represented communities at risk for serious disease, i.e., older adults, racial/ethnic groups, pregnant and immunocompromised persons, having equitable participation in research.
International bodies, like the International Conference on Harmonization (ICH) of Good Clinical Practice, provide guidance to nations. Also, WHO encourages all countries to have Independent National Immunization Technical Advisory Groups (known as NITAGs) to review the approved vaccine data according to the local epidemiology in order to determine who should or should not receive the vaccine. NITAGs build the capacity needed for global vaccine safety standards. After clinical trials, we continue to monitor vaccines for safety by using public health surveillance systems.
References
Dixon, J.R. The International Conference on Harmonization Good Clinical Practice guideline. Quality Assurance 1998; 6(2):65-74. doi: 10.1080/105294199277860.
Doshi, P. Pandemrix vaccine: why was the public not told of early warning signs? BMJ 2018;362:k3948 doi: 10.1136/bmj.k3948.
GAVI. Can vaccine clinical trials be sped up safely for COVID-19? https://www.gavi.org/vaccineswork/how-covid-19-leading-innovation-clinical-trials
Graham, J.E., Borda-RodrigeuzRodriguez, A., Huzair, F., and Zinck, E. Capacity for a global vaccine safety system: The perspective of national regulatory authorities. Vaccine, 2012; 30(33), 4953-4959. doi:10.1016/j.vaccine.2012 .05.045.
Graham, J.E. Smart Regulation: Will the government’s strategy work? Canadian Medical Association Journal 2005;173(12),1469-1470. doi: 10.1503/cmaj.050424. Retrieved from https://www.cmaj.ca/content/173/12/1469.short
Graham, J.E., and Nuttall, R. Faster Access to New Drugs: Fault Lines Between Health Canada’s Regulatory Intent and Industry Innovation Practices. Ethics in Biology, Engineering & Medicine – An International Journal 2013;4(3),231-239. doi: 10.1615/EthicsBiologyEngMed.2014010771. http://hdl.handle.net/10222/75934
Harmon, S.H.E., Faour, D.E., MacDonald, N.E., Graham, J.E., Steffen C., Henaff, L., and Shendale, S. The Global NITAG Network Survey Correspondents. Immunization governance: Mandatory immunization in 28 Global NITAG Network countries. Vaccine. https://doi.org/10.1016/j.vaccine.2020.09.053
Herder, M. and Graham, J.E. Opinion: Herder and Graham: Canadians need and deserve transparency on COVID-19 vaccines. Ottawa Citizen, September 15, 2020. https://ottawacitizen.com/opinion/herder-and-graham-canadians-need-and-deserve-transparency-on-covid-19-vaccines
Morten C.J., Kapczynski, A., Krumholz, H.M., and Ross, J.S. To help develop the safest, most effective coronavirus tests, treatments, and vaccines, ensure public access to clinical research data. Health Affairs Blog, March 26, 2020. https://www.healthaffairs.org/do/10.1377/hblog20200326.869114/full/
Why might a clinical trial be temporarily stopped?
The clinical trials are paused if a serious adverse event, like a heart attack, occurs to determine if it was caused by the vaccine.
What You Need to Know
- A clinical trial is paused if a serious adverse event occurs
- Any adverse event observed in a clinical trial is then assessed by independent experts to determine if the event is related to the vaccine
- If the event is not related to the vaccine, the clinical trial is resumed
Adverse Events in Clinical Trials
Large clinical trials are conducted on tens of thousands of participants. Pauses during such trials due to adverse events are not uncommon. They are treated as serious, and clinical trials are put on hold so that an investigation can be done. The intention is for experts to find out whether the observed adverse event was caused by the vaccine itself.
The clinical trial will remain on hold until it can be confirmed that the adverse event was not caused by the vaccine. An investigation can have one of two outcomes:
- If experts cannot say with certainty that the adverse event was not caused by the vaccine, the clinical trial is then permanently stopped, and individuals who have already participated in it are monitored for health issues.
- If experts can confirm that the adverse event was not due to the vaccine, meaning it is coincidental, the clinical trial resumes.
Reference
AstraZeneca and J&J get go-ahead to resume Covid-19 vaccine trials. Accessed on October 26, 2020, at https://www.ft.com/content/2d2d0e8c-3560-456f-bf3e-59a869e6aa00
Vaccine Introduction
What roles do front-line healthcare workers play in the safety and effectiveness of COVID-19 vaccines?
Front-line healthcare workers help by reporting adverse events and vaccine failure cases.
What You Need to Know
- Front-line healthcare workers report serious Adverse Events Following Immunization (AEFIs) and potential vaccine failures to Public Health Authorities
- Serious but very rare AEFIs are less common than the complications, hospitalization, and death that can result from having COVID-19
- AEFIs can be unrelated to the vaccine and completely coincidental
- Front-line healthcare workers track patients who are COVID-19 positive
- Healthcare providers help communicate the steps that are taken to determine if a serious AEFI was caused by the vaccine
Health Care Workers and Clinical Trials
The way a vaccine works is monitored during the entire vaccine process, starting with the different stages of research, known as clinical trials. These studies tell scientists if the vaccine is safe and whether it works. However, health authorities also need to know how well the vaccine performs in the real world. This information is gathered after the vaccine has been approved for use in the general population. Front-line healthcare workers are an important part of this process.
Front-line healthcare workers play a critical role by gathering information about the safety of vaccines and their effectiveness from the general public. They play an important part in reporting an Adverse Event Following Immunization (AEFI) and situations where a vaccine appears to have failed.
Reporting AEFIs
An Adverse Event Following Immunization, otherwise known as an AEFI, is any unexpected medical occurrence that happens after receiving a vaccine. If an AEFI occurs, either during clinical trials or after a vaccine has been approved for use, this does not necessarily mean that the vaccine caused it. The circumstances surrounding an AEFI must be thoroughly investigated to find out whether the new vaccine was indeed the culprit. AEFIs are often unrelated to the vaccination itself.
When front-line healthcare workers identify an AEFI, they gather important information about it, and then report that information to public health authorities.
Why the Information that Front-line Healthcare Workers Gather About AEFIs Is Important
The information that front-line healthcare workers gather about AEFIs helps public health authorities understand whether they are common or rare, and their level of severity. The more common AEFIs, which are not severe, usually resolve on their own. When a serious or rare AEFI is identified, public health authorities and the immunization program work hand in hand with front-line healthcare workers to ensure that there is clear communication while an investigation is carried out to determine if it was caused by the vaccine.
Reporting Vaccine Failures
Vaccine failure refers to situations where a vaccine does not work as well as it should to protect us against a specific disease. Vaccines can fail for two reasons. One is when our immune system does not produce enough antibodies after receiving the vaccination. The other is when our immune system does produce a lot of antibodies at first, but then cannot keep up a high level of antibodies over time. In both cases, the vaccine does not properly protect us against infection.
A vaccine’s effectiveness can be affected by many factors, such as age, underlying health conditions, gender, certain medications, nutrition and diet, and how the vaccine was stored and handled.
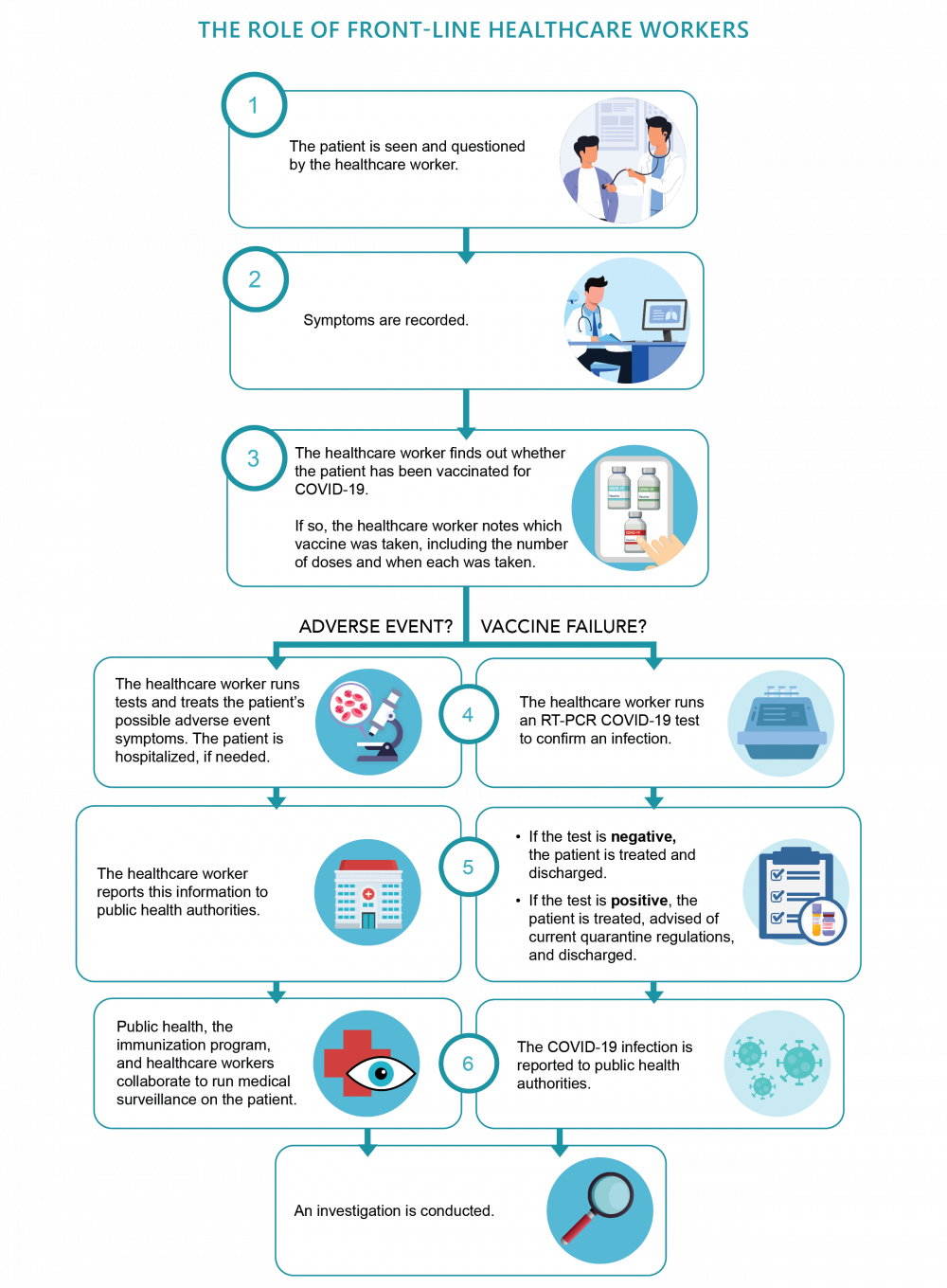
Front-line healthcare workers play an important role in determining vaccine effectiveness by reporting possible vaccine failures. When a patient appears to have COVID-19 symptoms, front-line healthcare workers take the following steps:
- They determine if the patient has received a COVID-19 vaccine. If so, healthcare workers note which vaccine was taken, including the number of doses and when each was taken.
- Then, they verify whether the patient actually tests positive for COVID-19 infection.
- Along with providing care for the patient, front-line healthcare workers report suspected vaccine failures—in other words, cases where a person has been vaccinated but still tests positive for COVID-19. This information is shared with Public Health Authorities for a follow-up investigation.
The investigation will determine if a vaccine failure has really happened and why. Vaccine failures can be for the following reasons:
- The vaccine itself was not able to provide the proper protection against COVID-19.
- The vaccine was not properly stored and handled, or there were problems with ingredients that were used to produce it, or any other vaccine program errors.
- The person who received the vaccine had underlying health conditions that may affect how their immune system has responded to the vaccine.
Further investigation can clarify the cause and provide more information on whether vaccine failures are being seen among the other people who received that specific vaccine.
More Information for Healthcare Providers
Adverse Effects Following Immunization (AEFIs) are rated based on severity:
- Less severe — the AEFI is mild, not life-threatening, and resolves on its own within a few days; or
- Serious — the AEFI is life-threatening or leads to death, hospitalization, significant disability, or congenital anomaly;
and on rarity:
- Common — the AEFI occurs in more than 1 person out of 1,000-10,000;
- Rare — the AEFI occurs in less than 1 person out of 1,000, but more than 1 person out of 10,000 people; or
- Very rare — the AEFI occurs in less than 1 person out of 10,000 people
Less Severe but More Common AEFIs
Clinical trials are designed to and can usually detect the less severe and more common AEFIs before approving a vaccine for use. COVID-19 vaccine clinical trials were able to detect common AEFIs (occurring in 1% to 10% of trial participants). For example, COVID-19 Phase I, II and III pre-licensure clinical trial data from a number of different COVID vaccines did not report any serious vaccine safety concerns according to peer-reviewed published reports. Less severe and more common AEFIs from COVID-19 vaccination include: a sore arm, poor appetite, redness at the site of injection, and mild fever. Since more common AEFIs are usually mild and resolve themselves without medical intervention, they do not stop a vaccine from getting approved by National Regulatory Authorities, unless they are deemed excessive.
Serious but Very Rare AEFIs
Serious but very rare AEFIs only occur in 0.01% of vaccinated people. These AEFIs are still less common than serious events due to COVID-19 infection, which include health complications, hospitalization, and death. The number of participants in most clinical trials is not big enough to detect serious but very rare AEFIs. When this type of AEFI is identified, public health authorities and the immunization program work hand in hand with front-line healthcare workers to ensure that there is clear communication while an investigation is carried out to determine what caused it.
As previously mentioned, clinical trials (before and after vaccine approval) are too small scale to detect these serious but very rare AEFIs and vaccine failures. In this regard, front-line healthcare workers are essential for detecting these events in the general population.
The education and empowerment of – and support to – health workers by their country’s immunization program is essential to a positive outcome in detecting and reporting suspected cases of COVID-19 vaccine serious AEFI and failures. They are central to the expansion of knowledge about the effectiveness and safety of the COVID-19 vaccines post-licensure.
- First, all front-line healthcare workers must know how to report a serious AEFI in their setting. It is then determined by an independent Causality Assessment Committee, in that country, if the reported adverse event was due to the vaccine, to a vaccine manufacturing error, an immunization program error, an immunization stress-related response, or if it was a coincidental unrelated event. This is done by using a systematic case review process. The World Health Organization (WHO) has developed a COVID-19 Vaccines: Safety Surveillance Manual to help countries carry out this important task.
- Second, all front-line healthcare workers collect information and report possible vaccine failures. As vaccine failure cases accumulate, differences in vaccine effectiveness might be seen with the different vaccines in different age groups as well as other subgroups and settings. This information is important locally, nationally, and globally. Depending on the findings, modification to the recommendations for use of the specific vaccine may need to be adjusted for different age groups and/or subgroups. This can include more doses, different intervals, the need for booster doses, etc. Ultimately, this information allows healthcare providers to offer more specific advice about the interval between receipt of vaccine doses and protection.
Moreover, each country shares their findings so that we can better understand the effectiveness of different COVID-19 vaccines within different settings. The assessment of AEFIs by the Causality Assessment Committee is also reported to the Uppsala Monitoring Centre, part of the World Health Organization Programme for International Drug Monitoring, including for vaccines.
For more information, visit: https://www.who-umc.org/
References
Assessing the Safety of COVID-19 Vaccines: A Primer. H, Petousis-Harris. 2020, Drug Safety, Vol. 43, pp. 1205-1210.
Considerations for diagnostic COVID-19 tests. Vandenberg, O., Martiny, D., Rochas, O. et al. October 14, 2020, Nat Rev Microbiol.
Immunization stress-related response – Redefining immunization anxiety-related reaction as an adverse event following immunization. Gold, M.S., MacDonald, N.E., McMurtry, M.C., Balakrishnan, M.R., Heininger, U., Menning, L., et al. 14, 2020, Vaccine, Vol. 38, pp. 3015-3020.
Lessons on causality assessment and communications from the 2019 South-East Asia Regional (SEAR) workshop on inter-country expert review of selected Adverse Events Following Immunization (AEFI) cases. MacDonald, N.E., Guichard, S., Arora, N., Menning, L., Wilhelm, E. 2019. Inter-country SEAR Workshop Participants, Communication Experts. 32, 2020, Vaccine, Vol. 38, pp. 4924-4932.
Optimizing safety surveillance for COVID-19 vaccines. Chandler, R.E. 8, 2020, Nature reviews. Immunology, Vol. 20, pp. 451-2.
Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. Dorjee, K., Kim, H., Bonomo, E., Dolma, R. 12, 2020, PLoS One, Vol. 15, p. e0243191.
Hospitalization and Mortality among Black Patients and White Patients with Covid-19. Price-Haywood, E.G., Burton, J., Fort, D., Seoane, L. 26, 2020, N Engl J Med., Vol. 382, pp. 2534-2543.
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. Polack, F.P., Thomas, S.J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., et al. December 10, 2020, N Engl J Med.
Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Voysey, M., Clemens, S.A.C., Madhi, S.A., Weckx, L.Y., Folegatti, P.M., Aley, P.K., et al. Dec 8, 2020, Lancet, pp. 99-111. Doi: S0140-6736(20)32661-1.
Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. Walsh, E.E., Frenck, R.W. Jr, Falsey, A.R., Kitchin, N., Absalon, J., Gurtman, A., et al. Oct 14, 2020, N Engl J Med.
The estimation of diagnostic accuracy of tests for COVID-19: A scoping review. Axell-House, D.B., Lavingia, R., Rafferty, M., Clark, E., Amirian, E.S., Chiao, E.Y. 2020, Journal of Infection, Vol. 81, pp. 681-697.
Three-month Follow-up Study of Survivors of Coronavirus Disease 2019 after Discharge. Liang, L., Yang, B., Jiang, N., Fu, W., He, X., Zhou, Y., et al. 47, 2020, J Korean Med Sci, Vol. 35, p. e418.
World Health Organization. Causality Assessment of an Adverse Event Following Immunization (AEFI) user manual for the revised WHP classification. Geneva: World Health Organization, 2018.
What is meant by benefit-risk assessment of the vaccine in high-priority populations?
A Benefit-Risk Assessment is used to weigh the positive and negative outcomes of vaccination in high-risk populations.
What You Need to Know
- Because vaccines are used to prevent diseases, the benefits of vaccination must be much greater than any risk of harm
- The Benefit-Risk Assessment of a vaccine is different for specific populations and people and can vary over time
- A vaccine is approved for use by the National Regulatory Authority only if there is a very positive benefit-risk balance
What are the Benefits?
The vaccine-preventable disease may cause no symptoms. But it can also cause mild, moderate, to severe disease, including long-term complications and even death. What the vaccine prevents in terms of negative results from the disease are known as the vaccine benefits.
What is Risk?
Risk is the possibility that danger or harm will occur. In the case of receiving vaccines, risk refers to the possibility that there would be some harmful effect from the vaccine. Possible risks from receiving COVID-19 vaccines are identified by continuously monitoring their use and identifying whether any harmful effects or safety issues happen and are due to the vaccine. No medical procedure or drug comes with zero risk. As a result, the potential risks of COVID-19 vaccines are compared to the known, serious risks to a person’s health if they become infected with COVID-19. The risks are also compared to the benefits from receiving the vaccine.
Benefits vs. Risks
Weighing the benefits and risks is known as a Benefit-Risk Assessment. The level of risk that we accept from vaccines is very low. This is because vaccines are given to healthy people, including young children and vulnerable people. It is essential that the benefits that a vaccine provides are significantly greater than any possible risk of harmful effects that may occur.
This type of Benefit-Risk assessment is based on scientific evidence and is what all regulatory decisions for vaccines are based on. A vaccine can only be approved for use by the National Regulatory Authority if the benefit-risk assessment shows that there are far more benefits than risks to using the vaccine. This is known as a positive benefit-risk balance.
A vaccine’s benefit-risk assessment might be different for specific groups of people, like children and immunocompromised people as compared to healthy adults. For example, some vaccines (like the inactivated influenza vaccine) do not work in infants under 6 months of age. Therefore, they are not recommended for that age group. The assessment may also be different for someone living in an area with an active disease outbreak because they are at a higher risk of being exposed to the disease.
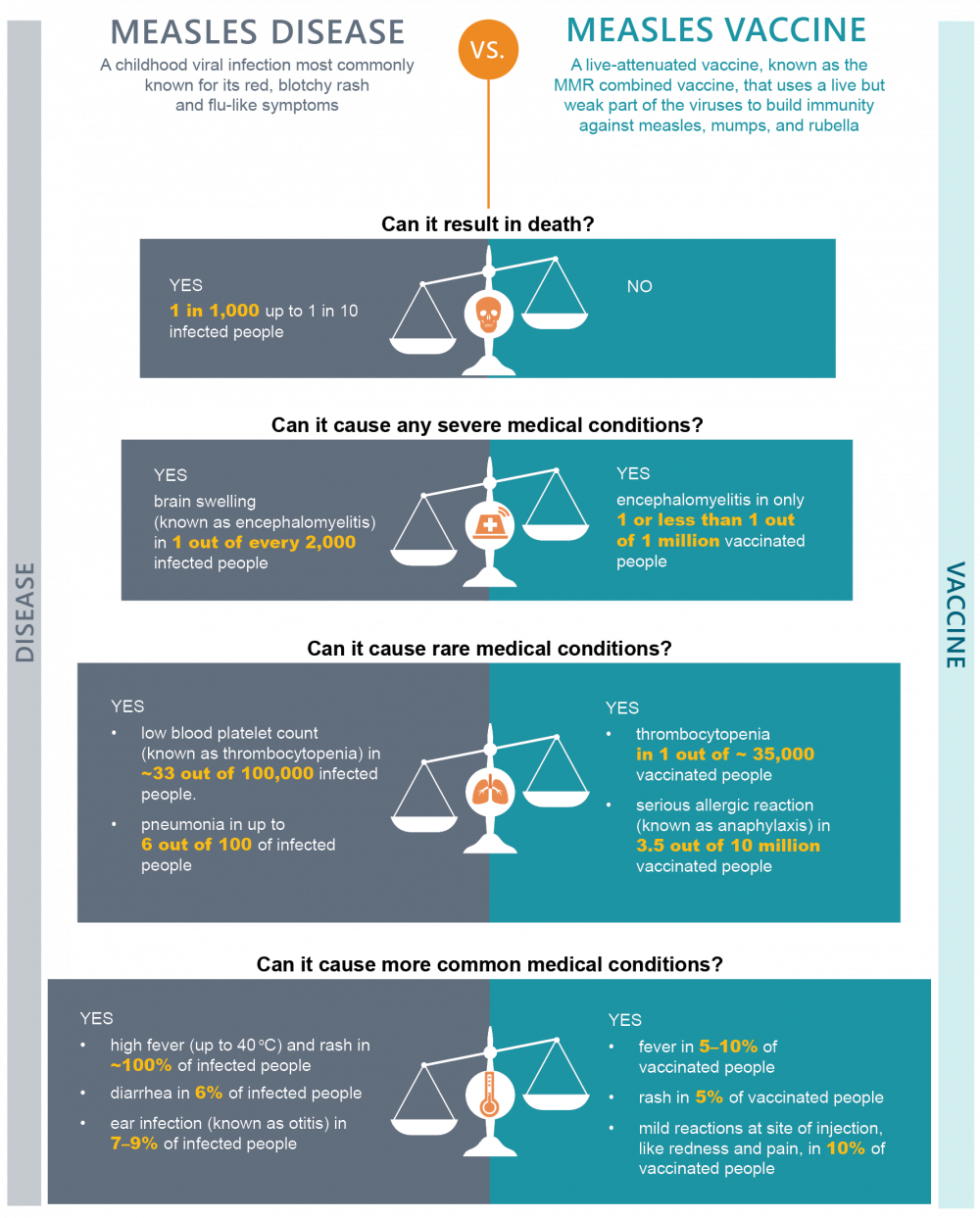
The following is an example of the benefit-risk assessment for the measles vaccine.
High-Priority Populations
High-priority populations are groups of people who are at a higher risk for a disease or more severe disease. These people would be made a priority for receiving a vaccine.
In the case of COVID-19, high-priority populations are groups of people who are at a higher risk of severe illness or death caused by COVID-19 disease, as well as an increased risk of exposure. Some examples of high-priority populations for COVID-19 vaccination include:
- Elderly people
- Immunocompromised people (which includes immunodeficient individuals, cancer patients, pregnant people, and those who are obese, have diabetes, hypertension, heart disease or chronic lung disease, and liver or kidney disease)
- Pregnant people
- Frontline healthcare workers
More information for healthcare providers about COVID vaccines
Children and Adolescents
It is recommended that children and adolescents aged 5 to 18 years have a complete 2-dose series followed by a booster dose 6 months later. COVID-19 vaccines (2- or 3-dose series) are authorized for children with no medical contraindications aged 6 months to 5 years. It is recommended that this age group be vaccinated using a complete 2-dose series of the Moderna (Spikevax) vaccine (with at least 8 weeks in between doses). Also, the COVID-19 vaccine should not be given on the same day as other (live or non-live) vaccines.
Children from 6 months to 11 years with any of the following characteristics can be at increased risk for severe outcomes of COVID-19, and vaccination is strongly recommended for these children:
- Medically fragile
- Obese
- Having medical complexities, with more than one comorbidity
- Having neurological disorders
- Having immune dysregulation associated with Down syndrome (trisomy 21)
- Other immunocompromising conditions
For young children 6 months to 5 years of age who are moderately to severely immunocompromised, the recommendation is that they be immunized with a 3-dose primary series of the Moderna (Spikevax) vaccine, allowing 4 to 8 weeks between each dose.
For children and adolescents 5 to 18 years of age who are moderately to severely immunocompromised, the recommendation is that they should be immunized with a 3-dose primary series (4 to 8 weeks between each dose) of a COVID-19 vaccine followed by a booster dose. The use of mRNA COVID-19 vaccines for booster doses is not currently authorized among children and adolescents (less than 18 years of age).
For adolescents 12 to 17 years of age who may be at higher risk of severe illness due to COVID-19, including those with one or more of the following conditions, the recommendation is that they should receive a complete 3-dose primary series of COVID-19 vaccine, followed by a booster dose within 6 months of receiving a complete series of doses:
- Cancer
- Chronic kidney disease
- Chronic lung diseases (like asthma)
- Cystic fibrosis
- Neurodevelopmental and other chronic neurological conditions including epilepsy and cerebrovascular disease
- Diabetes (type 1 and 2)
- Down syndrome (trisomy 21)
- Congenital heart disease (and other chronic heart diseases), including pulmonary hypertension
- Chronic liver disease
- Obesity (BMI of 30 or above)
- Pregnancy
- Sickle cell disease or thalassemia
- Substance use disorders
- Being immunocompromised, including primary immune deficiency, solid organ or haematopoietic stem cell transplant, HIV infection, and immunosuppressive therapy
- Medically fragile and having medically complex needs
Those previously infected with COVID-19: Although some protection may be conferred through a prior COVID-19 infection, vaccination after infection provides a stronger, more long-lasting, and broader protection. It is recommended that mRNA COVID-19 vaccines should be offered to these individuals (5 years of age and older). Again, the vaccination of younger children aged 6 months to 5 years of age is authorized.
Adults
It is recommended that all adults have a primary series, preferentially of an mRNA COVID-19 vaccine, followed by booster doses (for a total of 3-4 doses). Following the initial vaccine dose, subsequent doses should be given at 8-week intervals.
Some adults are at increased risk for serious COVID infection, which could lead to hospitalization and even death. These populations are urged to be immunized with a primary series and to receive one or two booster doses. Such individuals include:
- Older adults: 70 years and over (especially over 80 years of age)
- Pregnant and breastfeeding people
- Persons with an autoimmune condition: Autoimmunity can affect one’s immune system’s ability to fight a virus. It is recommended that an mRNA COVID-19 vaccine be given to individuals with an autoimmune condition.
- Immunocompromised persons: When an immune system is compromised, it cannot fight infectious disease as strongly as it should. For individuals who are moderately to severely immunocompromised, it is recommended that 3 doses of an mRNA COVID-19 vaccine be given.
Vaccination is also recommended for previously healthy adults with special circumstances, such as:
- Individuals previously infected with COVID-19: Although some protection may be conferred through a prior COVID-19 infection, vaccines after infection provides a stronger and long-lasting protection, and it is recommended that mRNA COVID-19 vaccines should be offered to these individuals (5 years of age and older).
- Travellers: Travellers have an increased chance of exposure. They should receive a complete series of COVID-19 vaccine at least 14 days prior to departure. Two weeks or more may be required to meet entry requirements for other countries.
- Persons new to Canada: Similar to travellers, newcomers may have increased risk of exposure and may not have received the type of COVID-19 vaccines approved in Canada. The Public Health Agency of Canada (PHAC) recommends that people who are planning to live, work, and/or study in Canada have a complete series of Health Canada-authorized COVID-19 vaccines with booster doses (for a total of 3-4 doses).
For more information, please visit: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-26-covid-19-vaccine.html#a6
References
Arlegui, H., Bollaerts, K., Salvo, F. et al. Benefit–Risk Assessment of Vaccines. Part I: A Systematic Review to Identify and Describe Studies About Quantitative Benefit–Risk Models Applied to Vaccines. Drug Saf (2020). https://doi.org/10.1007/s40264-020-00984-7
Benefit-risk assessment in drug regulatory decision-making. Draft PDUFA VI Implementation Plan (FY 2018-2022). U.S. Food and Drug Administration. March 30, 2018. Accessed on Sept 30, 2020 at https://www.fda.gov/media/112570/download
Booth, A., Reed, A.B., Ponzo, S., Yassaee, A., Aral, M., Plans, D., et al. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS One. 2021 Mar 4;16(3):e0247461. doi: 10.1371/journal.pone.0247461.
Curtin, F., Schulz, P. Assessing the benefit: risk ratio of a drug – randomized and naturalistic evidence. Dialogues Clin Neurosci. 2011;13(2):183-90. PMID: 21842615; PMCID: PMC3181998.
National Advisory Committee on Immunization (NACI). Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 months to 5 years of age. Public Health Agency of Canada. 14 July 2022. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-moderna-spikevax-covid-19-vaccine-children-6-months-5-years.html
Rémy, V., Zöllner, Y., Heckmann, U. Vaccination: the cornerstone of an efficient healthcare system. J Mark Access Health Policy. 2015; 3:10.3402/jmahp.v3.27041. doi:10.3402/jmahp.v3.27041
Risk scales: benefits of vaccines far outweigh the risks. WHO Europe. Accessed on Oct 9, 2020 at https://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/2017/risk-scales-benefits-of-vaccines-far-outweigh-the-risks-2017
What are the common side effects expected with the COVID-19 vaccines?
Common side effects include pain and/or redness and swelling at injection site, mild fever, fatigue, headache, and minor muscle and/or joint pain.
What You Need to Know
- As with most vaccines, common side effects of COVID-19 vaccines are usually mild and resolve by themselves
- Side effects are caused by the vaccine, while some adverse events following immunization may just be coincidental
What’s an Adverse Event Following Immunization?
A vaccine adverse event (also known as an Adverse Event Following Immunization or AEFI) is any unwanted medical occurrence that a patient experiences after receiving a vaccine. During clinical trials, even the placebo (saline) can cause AEFIs. An adverse event is not necessarily due to the vaccine. In many cases it is coincidental. Some AEFIs are caused by the vaccine itself and are known as side effects. Others may be related to the immunization procedure. AEFIs that are common or frequent are usually minor. Minor side effects after receiving a vaccine are not uncommon and can be attributed to the immune system being activated.
Minor AEFIs occur more commonly, usually within a few hours of injection. They self-resolve within a few days. Minor side effects include:
- Pain and/or redness and swelling at site of the injection. For example, this occurs in 15% of adults and 5% of children when receiving the hepatitis B vaccine.
- Mild fever (above 38°C, but below 39°C). For instance, this happens in 10% of people who receive the tetanus vaccine.
Sometimes, a more common AEFI (like swelling) may be considered severe: for example, a very swollen and red arm in a young child after receiving the hexavalent vaccine.
Possible Side Effects from Vaccines
Any vaccine can cause side effects. Most common vaccine side effects are minor and go away on their own within a few days. Examples of vaccines where this occurs include Bacillus Calmette-Guérin (BCG), hepatitis B, and haemophilus influenzae type B vaccines, among others.
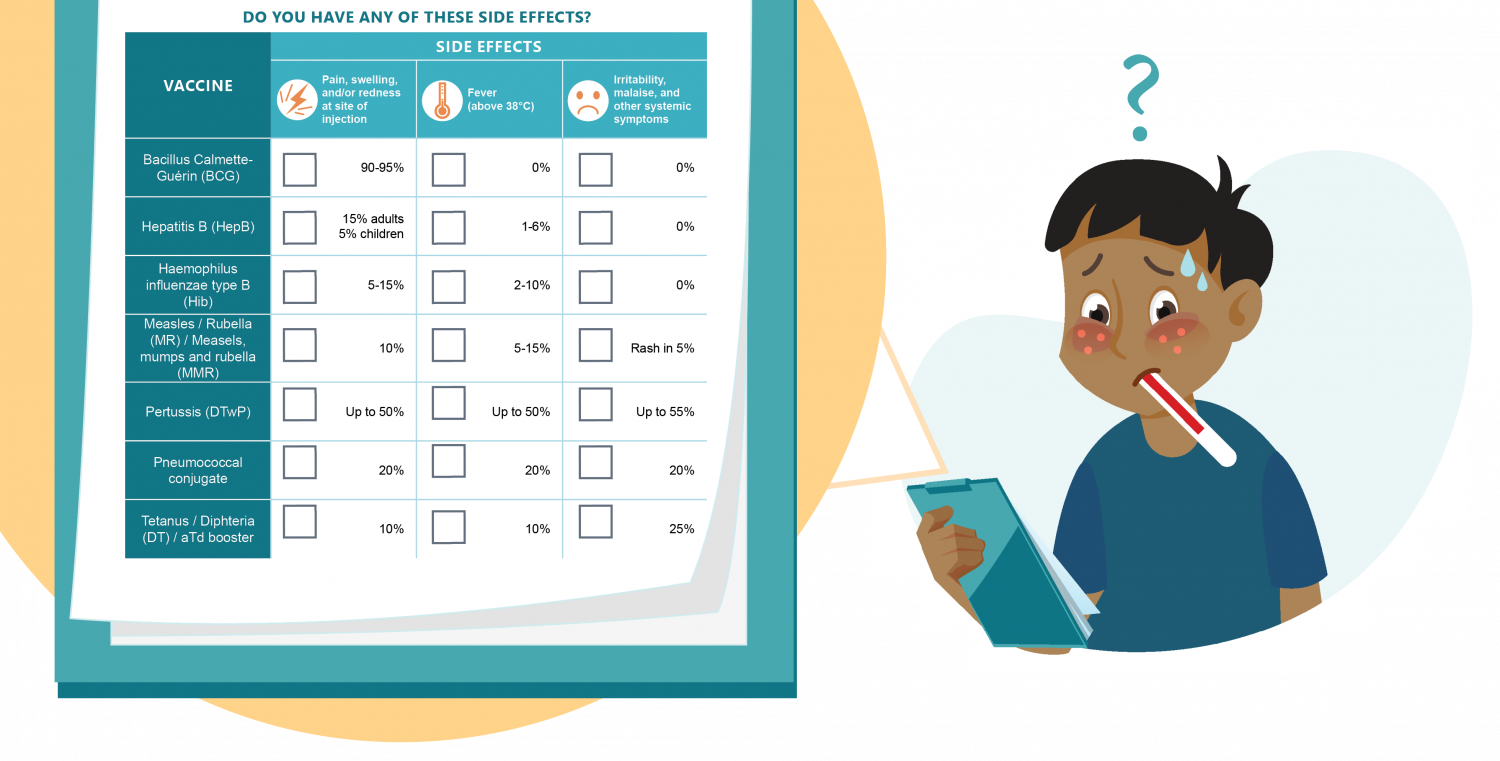
COVID-19 Vaccine Side Effects
Common, minor AEFIs after receiving an mRNA COVID-19 vaccine include:
- Fatigue (in 20% to 30% of adults)
- Headache (in 20% of adults)
- Minor muscle and/or joint pain (in 20% of adults)
Allergic Reactions to Vaccines
Allergic reactions are not the same as side effects. Although allergic reactions, like anaphylaxis, are possible, they are very rare. Allergic reactions happen within a few minutes after receiving a vaccine. They are usually caused by an allergy to a specific ingredient in the vaccine, like neomycin. Since anaphylaxis can be life-threatening, healthcare providers are always trained and prepared to treat it when giving a vaccine injection.
Serious Adverse Events Following Immunization
AEFIs that are common or frequent are usually minor. Serious AEFIs can occur but are very rare. An AEFI is only considered serious if it:
- Results in death
- Is life-threatening
- Causes a person to be hospitalized (or prolongs their hospitalization)
- Is persistent, meaning that it does not resolve by itself
- Causes significant disability and/or incapacity
- Results in a congenital anomaly and/or birth defect
- Needs medical intervention to prevent serious damage
You can learn more about AEFIs here.
More Information for Healthcare Providers
Phase III clinical trials for COVID-19 vaccines revealed very few adverse effects. Those that did occur were not serious. The vaccines were also deemed very effective in preventing hospitalization or death due to COVID infection.
Vaccine adverse events are classified as follows:
- Vaccine product-related reaction
- Vaccine quality-related reaction
- Immunization error-related reaction
- Immunization stress-related reaction
- Coincidental event
Redness, soreness, and swelling at the site of injection are normal for any injectable vaccine. Some other side effects have been confirmed with approved COVID-19 vaccines used in Canada:
-
The Pfizer (-BioNTech Comirnaty COVID-19) vaccine can cause headaches, chills, joint pain, fatigue, muscle aches, mild fever, and chills. These side effects are more common in younger recipients. Most side effects after the second dose were mild to moderate.
Rare but serious side effects include myocarditis and pericarditis (inflammation of the heart and/or heart tissues) and facial paralysis, known as Bell’s palsy.
-
The Moderna (Spikevax COVID-19) vaccine reported similar minor common side effects. More common side effects also include nausea, vomiting, irritability, sleepiness, and loss of appetite. It was noted that systemic adverse events were more common following the second dose and in those receiving the highest dose. Extensive swelling of the vaccinated limb has been spontaneously reported.
Rare but serious side effects include myocarditis/pericarditis and Bell’s palsy.
-
The AstraZeneca (Vaxzevria COVID-19) vaccine similarly may cause fatigue, joint pain, headache, mild fever, chills, and muscle aches. Tinnitus (persistent ringing in the ears) is an uncommon side effect. Also, paraesthesia (unusual tingling or crawling sensation in the skin) and hypoaesthesia (decreased feeling or sensitivity) were both spontaneously reported.
Rare but serious side effects include blood clots (with low levels of blood platelets), leakage from small blood vessels (known as capillary leak syndrome), and Guillain-Barré syndrome (where the body’s immune system damages nerve cells).
-
The Janssen ([Johnson & Johnson] COVID-19) vaccine can cause the same minor side effects as the AstraZeneca vaccine.
Rare but serious side effects include blood clots with low levels of blood platelets, capillary leak syndrome, and Guillain-Barré syndrome.
-
The Novavax (Nuvaxovid COVID-19) vaccine reported similar minor common side effects. More common side effects also include nausea and vomiting.
Rare but serious side effects include anaphylaxis (a severe allergic reaction) causing hives (bumps on the skin that are often very itchy); swelling of the lips, face, tongue, or airway; difficulty breathing; increased heart rate; loss of consciousness; sudden low blood pressure; and/or abdominal pain, vomiting and diarrhea. A few cases of paraesthesia (a tingling feeling or crawling sensation in the skin), hypoaesthesia (decreased feeling or sensitivity), and myocarditis/pericarditis have been reported.
-
The Medicago (Covifenz COVID-19) vaccine can cause chills, fatigue, joint aches, headache, mild fever, muscle aches, nasal congestion, sore throat, cough, nausea, and diarrhea.
Rare but serious side effects include anaphylaxis.
For more information, please refer to: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines.html
References
Anderson, E.J., Rouphael, N.G., Widge, A.T., Jackson, L.A., Roberts, P.C. et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 29 Sep 2020. doi: 10.1056/NEJMoa2028436.
European Medicines Agency. COVID-19 vaccines safety update. 14 July 2022. https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccines-safety-update-14-july-2022_en.pdf
Grech, V., and Calleja, N. Theoretical novel COVID-19 vaccination risk of rare and severe adverse events versus COVID-19 mortality. Early Hum Dev. 2020 Oct 1:105212. doi: 10.1016/j.earlhumdev.2020.105212
Pfizer says Covid-19 vaccine showed moderate side effects. https://www.clinicaltrialsarena.com/news/pfizer-covid-vaccine-tolerability-data/
WHO vaccine safety basics e-learning course. 18 Oct 2020. https://openwho.org/courses/vaccine-safety-basics
What happens when someone has an adverse event?
Minor adverse events are tracked while serious adverse events are treated immediately and reported to the Causality Assessment Committee.
What You Need to Know
- All serious AEFIs are investigated to find out if they were caused by the vaccine
- Minor AEFIs resolve themselves without medical intervention, while serious AEFIs are treated immediately
Adverse events after vaccination are known as Adverse Events Following Immunization (AEFIs). They are generally mild and resolve on their own. Common minor AEFIs (like mild fever and redness/swelling at the site of injection) are usually not reported to public health. However, some very rare AEFIs can be serious (see below). When an AEFI is serious, it is reported to public health and investigated to determine whether it is due to the vaccine or the immunization process, or not.
Very Common, Common, Uncommon, Rare, and Very Rare AEFIs
AEFIs are categorized based on how often they occur in a vaccinated population:
Very common AEFIs occur in less than 20% of vaccine recipients. Symptoms usually happen within a few hours of receiving the vaccine and resolve by themselves after a short period of time without any long-term effects. These are often expected reactions that show the immune system has been activated. Common minor AEFIs can include:
- Mild fever
- Muscle pain and body aches
- Tenderness, redness and/or swelling at the injection site
It is important to note that some minor AEFIs are also seen during clinical trials with the injection of saline (salt water solution) as a placebo. This is due to the immunization process, specifically caused by the needle being injected through the skin and muscle.
Reporting of AEFIs
With any new vaccine (like the COVID-19 vaccines), at first, all AEFIs, including minor ones, are reported by healthcare providers. National platforms that are open to the public, such as the Canadian National Vaccine Safety (CANVAS) Network, are also used to collect such information. Once minor AEFIs caused by a new vaccine are well known, they usually are no longer reported.
Serious AEFIs are always thoroughly investigated regardless of whether the vaccine is new or one with a long track record. This is done to find out for that individual patient whether the vaccine or the immunization procedure caused the serious AEFI or whether it was a coincidental event unrelated to immunization. The assessment to determine causality requires full information about the medical event, the vaccine, the circumstances during the immunization, and other information, such as whether the AEFI was seen during the clinical trial, has been reported before with this vaccine, etc.
Very Rare but Serious AEFIs
Serious AEFIs are urgently treated by healthcare workers.
An AEFI is considered serious if it is life-threatening, causes a person to be hospitalized, does not resolve by itself, affects a person’s ability to move or think clearly, causes birth defects, or needs medical care to prevent serious damage.
Serious AEFIs only occur in less than 1 in 10,000 people. Because they are so rare, they are usually not detected in the clinical trials, which generally study relatively small numbers of people. Serious AEFIs are immediately investigated to determine if they were caused by the vaccine itself, by the immunization process or if they are coincidental. Most are coincidental. If the serious AEFI is indeed caused by the vaccine, the recommendations on use of the vaccine may be changed.
More Information for Healthcare Providers
The pre-approval process for a vaccine is rigorous, to ensure common minor AEFIs are detected and that the vaccine is efficacious in preventing serious disease. High-quality manufacturing of the vaccine is also required. It is very rare that a serious AEFI is due to a problem with the vaccine.
Healthcare workers closely work with public health authorities and their institutions to ensure that accurate information is supplied and validated, and that misinformation is not perpetuated. Healthcare workers play a very important role in monitoring AEFIs, as they:
- Recognize and report serious AEFIs to public health authorities
- Provide immediate care for serious AEFIs
- Help gather information for the Causality Assessment Committee that will determine if the serious AEFI is due to the vaccine or the immunization procedure, or not (meaning it is coincidental)
- Remain objective in reaching any conclusion about the cause of the AEFI
- Communicate in a factual, effective, timely, and respectful manner
The local public health authority also has a responsibility to openly communicate about serious AEFIs, and steps taken to investigate and establish if they are indeed side effects of the vaccine.
References
Gold, M.S., MacDonald, N.E., McMurtry, C.M., Balakrishnan, M.R., Heininger, U., Menning, L., et al. Immunization stress-related response – Redefining immunization anxiety-related reaction as an adverse event following immunization. Vaccine. 2020; 38(14):3015-3020.
World Health Organization EUROPE. Crisis Communication Template (2017). Accessed Sept 24, 2020. https://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/2017/crisis-communications-plan-template-2017
World Health Organization. Vaccine Safety Basics e-learning course. Module 4: Surveillance. Causality Assessment of AEFIs. Assessed Sept 24, 2020. https://openwho.org/courses/vaccine-safety-basics
World Health Organization. Vaccine Safety Basics e-learning course. Module 3. Adverse events following immunization. Accessed Sept 24, 2020. https://openwho.org/courses/vaccine-safety-basics
Long term effects
Can COVID-19 vaccines affect future fertility and periods?
COVID-19 vaccines do not cause infertility or changes to periods.
What you need to know
- The idea that immunization causes infertility is a common rumour when any new vaccines are introduced.
- After millions of doses of COVID-19 vaccines have been given globally, there is no evidence that COVID-19 vaccines affect fertility, menstrual cycles, or puberty.
- COVID-19 mRNA vaccine clinical trials did not report any infertility either in men or in women.
Concerns about Immunization and Fertility Are Not New!
Concerns about immunization possibly affecting fertility are not new. This longstanding myth has been fueled by misinformation and has caused serious problems for public health. For example, in the early 2000s, this belief caused a boycott of the polio vaccine in Northern Nigeria. The public feared that the polio vaccine contained ingredients that caused infertility, yet this is false. Similar concerns were raised in Canada when the school-based vaccination program against human papillomavirus infection (HPV) started.
The evidence is clear that all vaccines, including the COVID-19 vaccines, that are given to prevent infectious diseases do not cause infertility. Regardless, this fear still exists! In fact, Google searches on COVID-19 vaccines and fertility/infertility were among the five most popular searches in February 2021.
COVID-19 Vaccines and Fertility
The COVID-19 mRNA vaccine clinical trials did not report any infertility issues. Studies have shown there is no change in sperm counts after COVID-19 vaccination. Further large studies have shown no change in fertility, menstruation, or puberty even after millions of doses have been given around the world. As before, with other vaccines, concerns about vaccination impacting fertility were unfounded rumours.
Responding to COVID-19 Vaccination Misinformation
In response to the vast misinformation on COVID-19 vaccination and fertility concerns, expert fertility organizations, like the American College of Obstetricians and Gynecologists, the American Society for Reproductive Medicine, the Society for Maternal-Fetal Medicine and the Society of Obstetricians and Gynaecologists of Canada, have issued strongly worded statements confirming that there is absolutely no evidence of the COVID-19 vaccine causing infertility. The British Fertility Society also developed guidance that includes the following highlights:
- There is no evidence of any kind that the approved COVID-19 vaccines can affect the fertility of women or men.
- Receiving the COVID-19 vaccine does not affect a person’s ability to donate eggs or sperm, nor to receive in vitro fertilization (IVF) treatment.
- Adults and adolescents should get vaccinated for COVID-19 because it is a serious disease with no cure.
COVID-19 Vaccination and Puberty
The North American Society for Pediatric and Adolescent Gynecology emphasizes that there is no evidence that COVID-19 vaccination affects puberty or growth. It is common to have varying menstrual cycles in the first few years after menses have started in adolescence. They also explain that irregular menstrual cycles after receiving a COVID-19 vaccine (or recovering from a COVID-19 infection) are not surprising because infections, immune reactions, and fevers are all known to cause short-term changes in menstrual cycles.
More Information for Healthcare Providers
Overall, the idea that COVID-19 vaccines have a negative impact on fertility and growth is incorrect. Experts advise adults and adolescents, as stated by the British Fertility Society, that there is “no evidence, and no theoretical reason, that any of the vaccines can affect the fertility of women or men.”
References
American College of Obstetricians and Gynecologists, the American Society for Reproductive Medicine, and the Society for Maternal-Fetal Medicine. News: Medical Experts Continue to Assert that COVID Vaccines Do Not Impact Fertility. American College of Obstetricians and Gynecologists. Feb 4, 2021. https://www.acog.org/news/news-releases/2021/02/medical-experts-assert-covid-vaccines-do-not-impact-fertility
Association of Reproductive and Clinical Scientists, British Fertility Society. COVID-19 vaccines and fertility. Feb 8, 2021. www.britishfertilitysociety.org.uk/wp-content/uploads/2021/02/Covid19-Vaccines-FAQ-1_3.pdf
Bowman, C.J., Bouressam, M., Campion, S.N., Cappon, G.D., Catlin, N.R., Cutler, M.W., et al. Lack of effects on female fertility and prenatal and postnatal offspring development in rats with BNT162b2, a mRNA-based COVID-19 vaccine. Reprod Toxicol 2021; 103: 28-35.
Diaz, P., Reddy, P., Ramasahayam, R., Kuchakulla, M., and Ramasamy, R. COVID-19 vaccine hesitancy linked to increased internet search queries for side effects on fertility potential in the initial rollout phase following Emergency Use Authorization. Andrologia 2021; June 28: e14156.
Gonzalez, D.C., Nassau, D.E., Khodamoradi, K., Ibrahim, E., Blachman-Braun, R., Ory, J., and Ramasamy, R. Sperm Parameters Before and After COVID-19 mRNA Vaccination. JAMA 2021;326(3):273-274.
Iacobucci, G. Covid-19: No evidence that vaccines can affect fertility, says new guidance. BMJ 2021; 372: n509.
Society of Obstetricians and Gynaecologists of Canada. SOGC statement on COVID-19 vaccination and fertility. Society of Obstetricians and Gynaecologists of Canada. March 18, 2021. https://www.sogc.org/common/Uploaded%20files/Latest%20News/EN_SOGCStatement_COVID-19Vaccination-Fertility.pdf
Talib, H., Berlan, E.D., and Tyson, N. NASPAG. NASPAG Advocacy Committee. North American Society for Pediatric and Adolescent Gynecology. May 2021. https://www.naspag.org/naspag-position-statement-on-covid-19-vaccines-and-gynecologic-concerns-in-adolescent-and-young-adults
Watson, R.E., Nelson, T.B., and Hsu, A.L. American Society for Reproductive Medicine. Fertility considerations: The COVID-19 disease may have a more negative impact than the COVID-19 vaccine, especially among men. Fertility and Sterility. American Society for Reproductive Medicine, March 19, 2021. https://www.fertstertdialog.com/posts/fertility-considerations-the-covid-19-disease-may-have-a-more-negative-impact-than-the-covid-19-vaccine-especially-among-men?room_id=871-covid-19
Yahya, M. Polio vaccines – "No thank you!". Barriers to polio eradication in Northern Nigeria. 2007, African Affairs 2007;106(423):185-204.
How would we know if a vaccine had long-term safety concerns?
Public health surveillance systems are used to monitor adverse events following immunization, for a long period of time.
What You Need to Know
- Adverse Events Following Immunization (AEFIs) are monitored for as long as a vaccine is used.
- Two different types of surveillance (active and passive) are used to monitor AEFIs of special interest.
National Regulatory Authorities Approve Vaccines and Monitor for Safety
National regulatory authorities, like Health Canada and the US Food and Drug Administration (FDA), ultimately approve vaccines based on safety and effectiveness. Once the vaccine is used in the general public, it continues to be monitored for any safety concerns and adverse events.
While initial clinical trials are done on healthy adults, scientists still need to understand how a vaccine affects other populations or age groups. Once approval is given by the National Regulatory Authority, pregnant people, immunocompromised individuals, adults with other underlying diseases, and children and adolescents may be studied in clinical trials. To detect very rare events, larger populations than those studied in clinical trials are needed. This is one of the reasons why surveillance for safety is carried out after the vaccine is made available to the general population.
Public Health Surveillance Systems
Public health surveillance systems are the eyes and ears of public health authorities. When a new vaccine is used, public health surveillance systems identify any specific adverse events of special importance following vaccination. COVID-19 vaccines are no exception! National and international public health organizations have put together guidelines and strategies to ensure that vigorous safety monitoring of COVID-19 vaccines is properly done.
Passive Surveillance
Passive surveillance systems are the most cost-effective way to monitor for adverse events. It is recommended that all countries use passive surveillance systems to collect reports of AEFIs from healthcare providers, vaccine manufacturers and the public. These reports are then compiled and analyzed to seek out any safety concerns with the vaccine. This would include, for example, a medical condition that is reported more often after vaccination than was expected.
Active Surveillance
Active surveillance can be much more expensive. It is recommended that countries that can do so, set up active surveillance systems that search for cases of adverse events after immunization of special importance. Adverse events of this nature would include seizures, Guillain-Barré Syndrome, blood clots (known as thrombosis), and others. Public health active surveillance systems also compare how such conditions show up in both vaccinated and unvaccinated groups to see whether the AEFI was caused by the vaccine itself or was simply coincidental.
Both passive and active surveillance of adverse events following immunization need to be assessed for causality – Did the vaccine or the immunization procedure cause the event, or was the event coincidental? This must be done rigorously and scientifically, and is done for all serious AEFIs, such as hospitalizations, deaths, etc. Note that “serious” and “severe” are not the same.

More Information for Healthcare Providers
In December 2020, the World Health Organization’s Global Advisory Committee on Vaccine Safety and an international consortium of vaccine safety experts, the Brighton Collaboration, joined forces with the Coalition for Epidemic Preparedness Innovations (CEPI) to establish guidelines and case definitions for monitoring the safety of COVID-19 vaccines. The COVID-19 vaccines: safety surveillance manual, published by the World Health Organization, also includes tools for vaccine safety surveillance.
References
Brighton Collaboration. 2022. Brighton Collaboration is working diligently to fight the coronavirus disease (COVID-19) pandemic. SPEAC – a CEPI Project. The Task Force for Global Health. Accessed on 11 July 2022. https://brightoncollaboration.us/covid-19/
Department of Immunization, Vaccines and Biologicals. 2012. Global Vaccine Safety Blueprint. Geneva: World Health Organization. Accessed on 5 October 2020. https://www.who.int/vaccine_safety/publications/en/
Nsubuga, P., White, M.E., Thacker, S.B., et al. Chapter 53. Public Health Surveillance: A Tool for Targeting and Monitoring Interventions. Disease Control Priorities in Developing Countries. 2nd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; New York: Oxford University Press; 2006.
2020. Global Advisory Committee on Vaccine Safety, 27-28 May 2020. Wkly Epidemiol Rec. 95:325-336. Accessed on 5 October 2020. https://apps.who.int/iris/bitstream/handle/10665/333136/WER9528-eng-fre.pdf?ua=1
Are COVID-19 vaccines safe in pregnancy and for those looking to become pregnant?
COVID-19 vaccines are safe before and during pregnancy.
What You Need to Know
- COVID-19 vaccines are safe and recommended before and during pregnancy.
- COVID-19 vaccines are effective at preventing severe illness, hospitalization, and death in pregnant people.
- COVID-19 infection during pregnancy can result in premature births, stillbirths, and death.
- COVID-19 vaccination offers protection against becoming severely ill during pregnancy and giving birth to pre-term babies.
Safety and Effectiveness of COVID-19 Vaccines in Pregnancy:
There is no evidence to suggest thatCOVID-19 vaccines pose a risk to the pregnancy or to the fetus. This is because COVID-19 vaccines do not contain the live virus that causes disease. This means that COVID-19 vaccines cannot cause infection in either pregnant people or their babies.
The following COVID-19 vaccines currently used in Canada have been approved for use in pregnant people (as well as in people trying to get pregnant):
- Pfizer-BioNTech vaccine
- Moderna Spikevax vaccine
- AstraZeneca Vaxzevria vaccine
- Janssen (Johnson & Johnson) Jcovden vaccine
- Novavax Nuvaxovid vaccine
The benefits of vaccination during pregnancy outweigh the potential risks. In fact, pregnancy poses a higher risk of severe illness caused by the COVID-19 virus. COVID-19 vaccines offer strong protection. They are especially recommended for pregnant people living in communities with high transmission of the COVID-19 virus and for pregnant people with higher individual risk of exposure or severe illness: for example, having pre-existing health conditions, a weakened immune system, or an occupation that necessitates dealing with the public, all while pregnant.
Approved COVID-19 vaccines have been found to be just as effective in pregnant people as they are in those who are not pregnant. COVID-19 vaccines are very effective in preventing severe illness, hospitalization, and death from COVID-19 during pregnancy. Some studies have also shown that antibodies against the COVID-19 virus can be transferred to the fetus through the umbilical cord. This means that vaccination during pregnancy can offer babies protection against COVID-19 as well.
The National Advisory Committee on Immunization (NACI) recommends that pregnant persons be vaccinated for COVID-19. Additionally, pregnant people and individuals who are breastfeeding should receive a COVID-19 booster regardless of the number of doses previously received (or the stage of pregnancy).
Pregnant People and their Babies Are at Higher Risk
Pregnant people are at a higher risk of developing severe illness due to COVID-19 and more likely to be hospitalized, requiring intensive care and ventilation as a result. Pregnant people are at a higher risk of dying (either during pregnancy or shortly after giving birth) due to COVID-19.
All pregnant people are at a higher risk of COVID-19 health complications. However, those who are older than 35, are overweight, and/or have an existing health condition (like diabetes or hypertension) have an even higher risk.
Pregnant people infected with COVID19 also have a higher risk of giving birth prematurely. Premature births increase the risk that babies will need intensive care. There is also a higher risk of the baby being stillborn.
COVID-19 Vaccination for Those Trying to Become Pregnant
People who are trying to become pregnant can receive COVID-19 vaccines. Since there is a higher risk of severe illness during pregnancy due to COVID-19, it is strongly recommended that people trying to become pregnant get vaccinated beforehand to better protect themselves and their babies.
COVID-19 vaccination does not affect fertility or the ability to become pregnant. Also, clinical trials found that vaccinated people are just as likely to conceive as non-vaccinated people.
The World Health Organization does not recommend delaying pregnancy or terminating pregnancy because of COVID-19 vaccination. Also, there is no need to test for pregnancy before receiving a COVID19 vaccine.
More Information for Healthcare Providers
Public health surveillance of pregnant people has been conducted since February 2022 in different countries and shows no safety concerns related to COVID-19 vaccination in pregnancy. For example:
- In the United States, over 198,000 pregnant people have been monitored, most of whom had received either the Pfizer or Moderna COVID-19 vaccine. No differences in the rate of pre-term births, stillbirth, and miscarriages between vaccinated and non-vaccinated pregnant people were found.
- In the United Kingdom, over 100,000 pregnant people have been monitored, most of whom had received mRNA COVID-19 vaccines. No differences in pregancyand birth outcomes between vaccinated and non-vaccinated pregnant people were found.
- In Brazil, over 1 million pregnant people have been monitored, most of whom had received the Pfizer COVID-19 vaccine (with a small portion having received the AstraZeneca COVID-19 vaccine). So far, no pregnancy-specific safety concerns have been found.
Clinical trials in pregnant people are now underway or planned for several COVID-19 vaccines, and monitoring of people who received vaccines during pregnancy, and their babies, is ongoing.
Animal studies, known as the Developmental and Reproductive Toxicology (DART) studies, also did not show any harmful effects on vaccinated, pregnant animals and their offspring.
References
Allotey, J., Stallings, E., Bonet, M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi:10.1136/bmj.m3320 (3 September 2020); updated 10 March 2021.
Ciapponi A, Bardach A, Mazzoni A, et al. Safety of components and platforms of COVID-19 vaccines considered for use in pregnancy: A rapid review. Vaccine. 2021;39(40):5891-5908. (6 June 2021).doi:10.1101/2021.06.03.21258283.
COVID-19 vaccine surveillance report – week 6. London: UK Health Security Agency; 2022. (10 February 2022). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1054071/vaccine-surveillance-report-week-6.pdf
Dagan, N., Barda, N., Biron-Shental, T., et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693-1695. doi:10.1038/s41591–021–01490–8 (9 September 2021).
Fu, W., Sivajohan, B., McClymont, E., et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int J Gynaecol Obstet. 2021. (5 November 2021). doi:10.1002/ijgo.14008.
Hillson K, Clemens SC, Madhi SA, et al. Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination. Lancet. 2021;398(10312):1683-4. doi: 10.1016/S0140-6736(21)02282-0 (21 October 2021).
Interim recommendations for use of the Bharat Biotech BBV152 COVAXIN® vaccine against COVID-Geneva: World Health Organization; 2021. (3 November 2021, updated 15 March 2022). https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-bbv152-covaxin
Kharbanda, E.O., Haapala, J., DeSilva, M., et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326(16):1629-1631. (9 September 2021). doi:10.1001/jama.2021.15494.
National Advisory Committee on Immunization (NACI). Updated guidance on COVID-19 vaccines for individuals who are pregnant or breastfeeding. An Advisory Committee Statement (ACS). (9 September 2022). https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/guidance-covid-19-vaccines-individuals-pregnant-breastfeeding.pdf
Observatório Obstétrico Brasileiro COVID-19 Vacinação. 2022. Accessed on 11 February 2022 at https://observatorioobstetrico.shinyapps.io/vacinacaocovid19/
Shimabukuro, T.T., Kim, S.Y., Myers, T.R., et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. (22 April 2021). doi: 10.1056/NEJMoa2104983.
Villar, J., Ariff, S., Gunier, R.B., et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–26. (23 April 2021). doi:10.1001/jamapediatrics.2021.1050.
Wei, S.Q., Bilodeau-Bertrand, M., Liu, S., et al. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193(16):E540-E548. (21 March 2021). doi:10.1503/cmaj.202604.
Wesselink AK, Hatch EE, Rothman KJ, et al. A prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. Am J Epidemiol. 2022. (22 January 2022). doi: 10.1093/aje/kwac011
Zauche, L.H., Wallace, B., Smoots, A.N., et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385(16):1533-1535. (9 September 2021). doi:10.1056/NEJMc2113891
Do COVID-19 vaccines work in immunocompromised or HIV-positive patients?
COVID-19 vaccines can be given to these patients.
What You Need to Know
- People living with HIV or who are immunocompromised are at a higher risk for severe illness due to COVID-19.
- COVID-19 immunization is recommended for HIV-positive and immunocompromised patients.
- Other COVID-19 prevention measures are highly recommended for people with poorly controlled HIV and for those who are severely immunocompromised.
COVID-19 Vaccines in HIV-positive People
At the beginning of the COVID-19 pandemic, different kinds of vaccines were being rapidly developed, which gave rise to concerns for using certain COVID-19 vaccines in people living with HIV. These concerns were due to HIV being a viral disease that kills CD4 white blood cells, weakening the immune system.
Although there is limited safety data for COVID-19 vaccines in patients with well-controlled HIV, what data we have so far is reassuring. Information for three COVID-19 vaccines approved in Canada for people living with HIV includes:
- Pfizer-BioNTech vaccine: no safety issues have been reported in patients with well-controlled HIV.
- Moderna vaccine: information is the same as that for the Pfizer-BioNTech vaccine.
- Janssen (Johnson & Johnson) vaccine: no added safety issues were noted in the large clinical trials that had participants with well-controlled HIV.
COVID-19 vaccine safety and effectiveness data for HIV patients who have poorly controlled HIV is lacking. In July 2021, the World Health Organization’s Strategic Advisory Group of Experts (WHO SAGE) developed an interim guidance document that mentions that the available data are too sparse to properly assess vaccine efficacy or safety for persons living with HIV who have poorly controlled HIV.
COVID-19 Vaccines in Immunocompromised People
Safety and effectiveness data for COVID-19 vaccines used in Canada are lacking for immunocompromised people. One of the challenges in collecting this data is that this is a very diverse group of people. For example, a person can be immunocompromised due to an illness that affects their immune system, or because of immunosuppressive medications used as treatment for medical conditions. As a result, WHO SAGE has noted that since there is not much available data, it is not possible to fully assess COVID-19 vaccine safety or possible risks in immunocompromised people. WHO SAGE also notes that in these people, the immune response to the vaccine may be reduced, which would reduce the vaccine’s effectiveness. In fact, COVID-19 vaccines appear to be ineffective in transplant patients, since their immune systems are severely suppressed by medications to avoid rejection of transplanted tissues.
Immunocompromised persons who are part of a group recommended for COVID-19 immunization should be vaccinated.
Other Prevention Measures Are Highly Recommended
Even if an HIV-positive or immunocompromised person has received a complete series of COVID-19 vaccine doses, other prevention measures are strongly recommended. This is because COVID-19 vaccines may not be as effective in severely immunocompromised persons or in people with poorly controlled HIV. Other prevention measures include:
More information on COVID-19 prevention and risk can be found at: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/prevention-risks.html
More Information for Healthcare Providers
The WHO SAGE on Immunization has developed guidance for policy makers on COVID-19 vaccines.
References
Coburn, S.B., Humes, E., Lang, R., Stewart, C., Hogan, B.C., et al. 2022. Analysis of Postvaccination Breakthrough COVID-19 Infections Among Adults with HIV in the United States. JAMA Netw Open, 2022 Jun 1; 5(6): e2215934. doi: 10.1001/jamanetworkopen.2022.15934. Accessed on 5 October 2022 at https://pubmed.ncbi.nlm.nih.gov/35671054/
Nault, L., Marchitto, L., Goyette, G., Tremblay-Sher, D., Fortin, C., et al. 2022. Covid-19 vaccine immunogenicity in people living with HIV-1. Vaccine, 2022 Jun 9; 40(26): 3633-3637. doi:10.1016/j.vaccine.2022.04.090. Accessed on 5 October 2022 at https://pubmed.ncbi.nlm.nih.gov/35568588/
World Health Organization (WHO). 2021. Interim recommendations for an extended primary series with an additional vaccine dose for COVID-19 vaccination in immunocompromised persons. Interim Guidance. Agenda, Policy & Strategy, Immunization, Vaccines and Biologicals, Strategic Advisory Group of Experts on Immunization, WHO Headquarters (HQ). (25 October 2021). Accessed on 5 October 2022 at https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-immunocompromised-persons
Contributors
Sonali Kochhar, MD1, Eve Dubé, PhD2, Janice Graham, PhD3, Youngmee Jee MD, PhD4, Ziad A. Memish, MD5, Lisa Menning6, Hanna Nohynek, MD, PhD7, Daniel Salmon, PhD8; Karina Top, MD, MS9, Noni E MacDonald, MD10
Affiliations and Conflict of Interest (COI)
-
Clinical Associate Professor, Department of Global Health, University of Washington, Seattle; Medical Director, Global Healthcare Consulting
COI - Nothing to declare -
Senior researcher at Quebec National Institute of Public Health; Invited Professor in Anthropology, Laval University, Canada
COI - Nothing to declare -
University Research Professor and Professor of Pediatrics (Infectious Diseases) and Anthropology, Dalhousie University, Canada
COI - Nothing to declare -
Visiting Professor, GSPA, Seoul National University; Special Representative for Health Diplomacy, Korea Foundation
COI - Nothing to declare -
Senior Infectious Disease Consultant, Director Research and Innovation Center, King Saud Medical City, Ministry of Health, Riyadh, Saudi Arabia
COI- Nothing to declare -
Technical Officer, WHO HQ Department of Immunization, Vaccines, and Biologicals, Geneva
COI - Nothing to declare -
Chief Physician, Deputy Head of Unit, Finnish Institute for Health and Welfare THL, Finland
COI - My institute THL has got a strategy of public private partnership; neither myself nor my unit receives such private funds/grants. -
Director and Professor, Institute for Vaccine Safety, Johns Hopkins University School of Public Health, United States
COI - Consulting and/or grants with Merck, Jenssen and Walgreens. -
Associate Professor of Pediatrics and Community Health & Epidemiology, Dalhousie University, Canada
COI - In the past 3 years I have received consultancy fees from Pfizer and grants from GSK to my institution for projects unrelated to this work. -
Professor Paediatrics, Dalhousie University, IWK Health Centre, Canada
COI- Nothing to declare
The CONSIDER Working Group
In recognition of the critical importance of COVID-19 vaccines and the need to understand their safety, the CONSIDER (COvid-19 vacciNe Safety questIons anD hEalthcare pRoviders) Working Group (WG) was created in September 2020.
The CONSIDER WG aims to provide clear, comprehensive answers to questions pertaining to COVID-19 vaccine safety prior to, and during the vaccines roll out to
1) facilitate scientific discussion between stakeholders, including front line health workers with potential vaccine recipients and
2) increase comprehension and transparency of information to facilitate acceptance and uptake.
There are likely to be concerns associated with COVID-19 vaccines given the accelerated pace of vaccine development, mistrust of the pandemic response by governments and growing suspicion regarding vaccines in some population groups.1-4 As for all new vaccines, there is the possibility that rare adverse reactions will not be identified in clinical trials and only found once a large number of people are vaccinated. Additionally, when vaccinating large number of people, some of them will have adverse health outcomes shortly after vaccination by chance alone. COVID-19 vaccine safety concerns need to be urgently addressed. The CONSIDER group was developed to provide responses to common COVID-19 vaccine safety questions that are understandable and accessible to key stakeholders.
Each answer is written by an expert member of the WG and reviewed by two or more other members.
Science is evolving rapidly. As more questions come to the group’s attention or more information becomes available (from COVID-19 vaccine clinical trials and early experience with vaccine introduction in countries), the answers will be updated and new answers will be posted. The questions and answers are hosted on this page and are cross-referenced on other sites.
The information provided on this page is written by an international group of experts in immunization and is applicable globally, including in the Canadian context.
Below is the latest version of the answers. This webpage will be updated as new answers are added.
References
- Schaffer DeRoo S, Pudalov NJ, Fu LY. Planning for a COVID-19 Vaccination Program. JAMA. 2020; 323(24):2458-2459. doi:10.1001/jama.2020.8711
- Lancet COVID-19 Commissioners, Task Force Chairs, and Commission Secretariat. Lancet COVID-19 Commission Statement on the occasion of the 75th session of the UN General Assembly. Lancet. 2020:S0140-6736(20)31927-9. doi: 10.1016/S0140-6736(20)31927-9.
- Kochhar S, Salmon DA. Planning for COVID-19 vaccines safety surveillance. Vaccine. 2020 Sep 11;38(40):6194-6198. doi: 10.1016/j.vaccine.2020.07.013
- MacDonald NE, Dube E. Vaccine safety concerns: Should we be changing the way we support immunization? EClinicalMedicine. 2020 Jun; 23:100402. doi: 10.1016/j.eclinm.2020.100402.
